Solitaire™ X Revascularization device
Solitaire™ X is a revascularization device designed for effective clot retrieval during thrombectomy procedures for acute ischemic stroke patients.
Find out more
Overview
The Solitaire™ device has become the most-published stent retriever with over 200 publications demonstrating clinically proven, tried-and-true performance.1,2
The Solitaire™ X device has a unique parametric design that has been fundamental to the generations of the Solitaire™ portfolio.
- This overlap design allows the stent to expand and compress in the vessel during deployment and retrieval.
- The distinctive, evenly spaced platinum markers enable accurate alignment and feedback during the procedure with a three-dimensional perspective.3*
Comprehensive Acute
Ischemic Stroke Portfolio
Solitaire™ X is integral in Medtronic’s wide-ranging AIS portfolio, offering responsive and complete solutions to better support your AIS patients.
Solitaire™ X works alongside a collection of agile products to deliver combination therapy as a proven data-backed approach.1
The benefits are clear with a 43.3% mTICI 2c-3 single pass using the combination technique.1 Our AIS portfolio features state-of-the-art products to enhance your AIS clinical offering:
- Phenom™ 21 and Phenom™ 27 Catheter
- React™ 68 and React™ 71 Catheter
- Riptide™ Aspiration System
- Cello™ Balloon Guide Catheter†
See the Neurovascular Product Catalog for more information.

Product details
Conform to anatomy with wall apposition and dynamic clot integration4,5*
- Maintains consistent cell size and structure over varying vessel diameters6*
- Provides multiple planes of contact to integrate with the clot, even double layering in smaller vessels7*
Percent overlap by vessel diameter5*

Visualize accuracy with real-time imagery
- Meaningful visibility with real-time visualization of the radiopaque markers
- Evaluate clot composition through body marker integration into the clot
- Visualize the expansion and compression of the stent to help identify clot characteristics8


Images courtesy of Dr. Alejandro Tomasello Weitz
Encapsulate the clot without damaging the wall9†
Differentiated radial outward force promotes clot and vessel wall contact during retrieval with the optimal amount of radial force.10*



Clinical evidence
Optimize outcomes by minimizing the number of passes11-13
A large real-world patient cohort demonstrated a first pass effect (FPE) rate of 40.5% and a modified FPE (mFPE) rate of 58.9% across patients treated with the Solitaire™ device.14‡
Achieve the clinical outcomes you expect
A large real-world patient cohort demonstrated the following results with the Solitaire™ device.15
87.9%
Successful reperfusion
(mTICI 2b-3, core-lab adjudicated)
56.5%
modified Rankin Scale (mRS) 0–2 at 90 days
1.4%
Symptomatic intercranial hemorrhage (sICH)
Ordering information
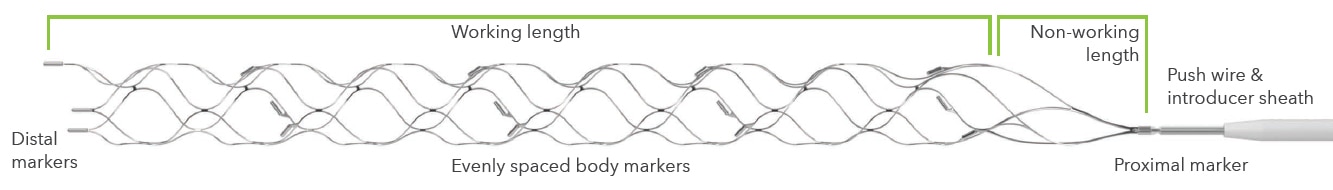
Solitaire X revascularization device portfolio information16
| Model | Recommended Vessel DiameterA (mm) | Microcatheter ID Range | Push Wire Length | Stent Diameter | Usable LengthB | Stent Length | Length from Distal Tip to Flourosafe Marker | Radiopaque Markers | Radiopaque Stent Markers Spacing | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (min) | (max) | (min–max) | (cm) | (mm) | (mm) | (mm) | (cm) | Distal | Prox. | (mm) | |
| SFR4-3-20-10 | 1.0 | 3.0 | 0.017″–0.027″ 0.43 mm–0.69 mm |
200 | 3.0 | 20.0 | 30.6 | <150 | 3 | 1 | 10 |
| SFR4-3-40-10 | 1.0 | 3.0 | 0.017″–0.027″ 0.43 mm–0.69 mm |
200 | 3.0 | 40.0 | 51.6 | <150 | 3 | 1 | 10 |
| SFR4-4-20-05 | 1.5 | 4.0 | 0.021″–0.027″ 0.53 mm–0.69 mm |
200 | 4.0 | 20.0 | 31.0 | <130 | 3 | 1 | 5 |
| SFR4-4-40-10 | 1.5 | 4.0 | 0.021″–0.027″ 0.53 mm–0.69 mm |
200 | 4.0 | 40.0 | 50.0 | <130 | 3 | 1 | 10 |
| SFR4-6-24-06 | 2.0 | 5.5 | 0.021″–0.027″ 0.53 mm–0.69 mm |
200 | 6.0 | 24.0 | 37.0 | <130 | 4 | 1 | 6 |
| SFR4-6-40-10 | 2.0 | 5.5 | 0.021″–0.027″ 0.53 mm–0.69 mm |
200 | 6.0 | 40.0 | 47.0 | <130 | 4 | 1 | 10 |
A. Based on smallest vessel diameter at thrombus site.
B. Usable length that is at least as long as the length of the thrombus.
Up to 3 flow restoration recoveries16
For a compatible microcatheter to help you smoothly navigate through even the most complicated anatomy, choose from the Phenom™ 21 or 27 catheter to deliver the Solitaire™ X device.
Manuals and technical guides
To view this technical manual please follow the link below
Visit the manualsAIS portfolio
See our stroke products, from stent retrievers to aspiration systems.
Based on bench testing results. Bench testing may not be representative of actual clinical performance.
Based on bench and animal testing results. Bench and animal testing may not be representative of actual clinical performance.
FPE defined as mTICI2c/3; modified FPE defined as mTICI 2b-3.
* Thrombectomy + IV-tPA
** Thrombectomy alone
† Cello is a trademark of and is manufactured by Fuji Systems Corporation.
References
Medtronic Data on File. Solitaire Literature Review Aug2022. Includes Solitaire FR, Solitaire 2, Solitaire Platinum, Solitaire X.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731.
Medtronic internal report. TR-NV12692 Rev A. Solitaire Platinum Competitive Testing. 07/04/2016
TR07-128B
Medtronic internal report. TR-NV13807 Rev A Calculating the Overlap Area in Solitaire Stents. 11/05/2017, Medtronic internal report. TR-NV15666A Rev A Solitaire 4 Revascularization Device Adoption Report. 08/10/2018, Medtronic internal report. D00419703 Rev A Marketing Claims Report Solitaire MAX Marketing Claims Testing and Evaluation. 26/02/2021, Medtronic internal report. D00324045 Rev A Solitaire MAX GLP Animal Study Design Validation Report. 28/01/2021
Medtronic internal report. TR-NV12554 Rev A. Marketing Evalaution and Claims of the Solitaire Platinum Device with Comparison to Other Device. 29-Mar-20177
Medtronic internal report. TR-NV13807 Rev A Calculating the Overlap Area in Solitaire Stents. 11/05/2017, Medtronic internal report., Medtronic internal report. D00419703 Rev A Marketing Claims Report Solitaire MAX Marketing Claims Testing and Evaluation. 26/02/2021
Tomasello A. The best of both worlds: Combination therapy for ischemic stroke. Oral presentation at: International Stroke Conference; February 9, 2022; New Orleans, LA.
Medtronic internal report. D00188173 Rev B. Product Specification Rational Report - Solitaire MAX Specification Rationale. 23-Apr-2020.
Medtronic internal report. D00419703 Rev B. Marketing Claims Report Solitaire MAX Marketing Claims Testing and Evaluation. 11-Nov-2022
García-Tornel Á, Requena M, Rubiera M, et al. When to Stop [published correction appears in Stroke. 2020 Jun;51(6):e118]. Stroke. 2019;50(7):1781-1788.
Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: A new measure for stroke thrombectomy devices. Stroke. 2018;49(3):660-666.
Flottmann F, Leischner H, Broocks G, et al. Recanalization rate per retrieval attempt in mechanical thrombectomy for acute ischemic stroke. Stroke. 2018;49(10):2523-2525.
Jadhav AP, Desai SM, Zaidat OO, et al. First pass effect with neurothrombectomy for acute ischemic stroke: Analysis of the systematic evaluation of patients treated with stroke devices for acute ischemic stroke registry. Stroke. 2022;53(2):e30-e32. Includes Solitaire FR, Solitaire 2.
Mueller-Kronast NH, Zaidat OO, Froehler MT, et al. Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS registry. Stroke. 2017;48(10):2760-2768.
Solitaire X Revascularization Device IFU: M009589CDOC2 (eIFU: M009589CDOC4).
See the device manual for detailed information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential adverse events. For further information, contact your local Medtronic representative and/or consult the Medtronic website at medtronic.eu
